Indigo Dye and its Derivatives
Categories:
Time of issue:
2023-03-31
INDIGO DYE AND ITS DERIVATIVES
Indigo naturalis has some chemical properties. Indigo remains the main component of Indigo naturalis. Indigo derivatives have different shades and colors: yellow, green, brown and violet. Together with indirubin (red) and their derivatives, they are the constituents of a preparation called Indigo naturalis. In Chinese medicine, it is commonly used as a heat remover to treat various ailments. The preparation known as Qing Dai may be produced from indigo plants like Polygonum tinctorium Ait., Isatis tinctoria Ait. and Baphicacanthus cusia Brem.
Indigo PLANT SOURCES
One particular species of indigo plant is Polygonum tinctorium Ait., an annual perennial from the Polygonaceae family. The species habitat resides predominantly in China and India. Its Latin name is derived from the type of knotweed (Polygonum) and recently it has been classified as a species which belongs to the Persicaria genus. The species can reach up to 80 cm in height. Its stem usually has a reddish color and is characterized by leaves which are narrowed at the base and are embedded on stalks. This plant is distinguishable by its pink or red flowers with white perianths. These flowers are grouped together in the form of short, thick ears, forming a loose flower panicle. In addition, this plant has compact, egg-shaped bundles in leaf axils and a fruit which forms a shiny nut. Baphicacanthus Cusi (Nees) Bremek., syn. Strobilanthes Cusi (Nees) is another plant species from which indigo is extracted. Kuntze is a perennial plant reaching 60 cm in height. It grows in clay and wet soils and is tolerant to different soil pH levels. The species blooms well in either partial or complete shade and has oval-shaped leaves and hermaphrodite flowers. Isatis indigotica Fort. is a species, commonly known as ëWoadî, whose habitat is found in steppe areas of the south-eastern Europe, the Caucasus and Asia Minor. Today, this species can be found in wild crops across almost all of Europe and in parts of Algeria and Morocco. This species can reach a height of 50-140 cm and is distinguishable by its characteristically bluish color. The lower stem leaves are oval shaped and are located on stalks, while the upper leaves are seated and cover the stem. Isatis indigotica Fort. is characterized by many yellow flowers gathered in inflorescences. Its petals are almost two times longer than the sepals, the cup has four sepals and the four-lobed crown contains six stamens and one pistil. Fruits form in single pods of up to 2.5 cm in length. Its color transitions from a dark shade to black violet, while it ripens. The species can often be found growing in chalk soils and around cliffed areas. Hot days and warm night temperatures, together with humid conditions accelerate the degradation and extraction processes. In tropical climates, freshly harvested plants are kept in water until the degradation and extraction of indigo water has occurred. The cultivation of indigoferous plants was also introduced into Europe and Japan. Here, harvested leaves are dried before being combined with water. Then, the leaves are composted in order to increase the concentration of the compound.
Currently the indigo naturalis in China is mainly extracted from Baphicacanthus Cusia leaf and stem.
INDIGO AND ITS DERIVATIVES
Dyes are colored substances, which have a chemical affinity to the substrate to which they are applied. They appear colored, because they absorb some wavelengths of light from the spectrum better than others. Pure indigo is only slightly soluble in water, making it ideal for use as a pigment. Originally, its use as a dye arose due to the reduction reaction. White indigo is formed as a result of indigo under going the reduction reaction. White indigo properties offer a better solublility than the one of the original substance, facilitating the dyeing of clothing and other textile materials. Its reduced form may be re-oxidized into substances of intense deep blue color, as a result of being left in contact with the open air. Indigo production decreased sharply in the mid-twentieth century only to rise again as a result of indigo being used as a colorant of denim. The demand for indigo increased to more than twenty tons in 2003. Indigo may also be mixed with a variety of other substances, resulting in a chemical reaction to obtain coloring pigments with green, blue and violet shades. A pharmacologically important isomer of blue indigo, present in the indigoferous plants is indirubin. It is red in color, however it is not used in the textile industry, just like the former compound. There are also other colorants derived from the other compounds, which constitute the pigment of different colors. Tyrian purple ñ 6,6-dibromoindigo, is a naturally occurring substance isolated from old sea shells. Currently, its use remains highly limited. 5,7,5, 7-Tetrabromo-derived indigo (blue Vat Blue 4B) an analogue of bisulfonic acid (cyan Blue Saxon) is used to dye textiles blue. Dye mixtures are also produced, which contain indigo as a constituent, for example indigo in the form of anthrone in Vat Blue.
Chemical characteristic of indigo
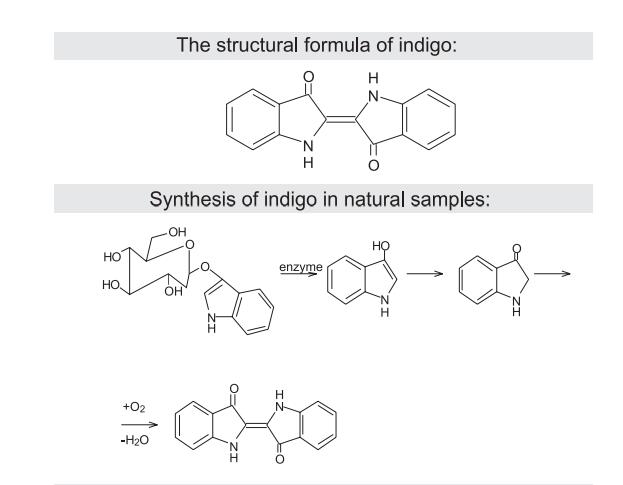
The substance has a dark blue color referred to the last visible shade of blue in the spectrum before the transition into purple. The attributed color index for indigo is C. I. Pigment Blue 66 I C. I. 7300. Chemical properties Indigo is a dark blue crystalline powder that sublimes between a temperature of 390 and 392OC. This alkaloid is insoluble in water, alcohol and diethyl ether, but is soluble in DMSO, chloroform, nitrobenzene and concentrated sulfuric acid. Indigo has the chemical formula of C16H10N2O2 and a molecular weight of 262.26. The molecule absorbs light in the orange part of the spectrum (λmax = 613 nm). This compound has its deep color thanks to the conjugation of double bonds which are adjacent to each other. Thus, the molecule has a planar structure.
Chemical characteristic of indirubin
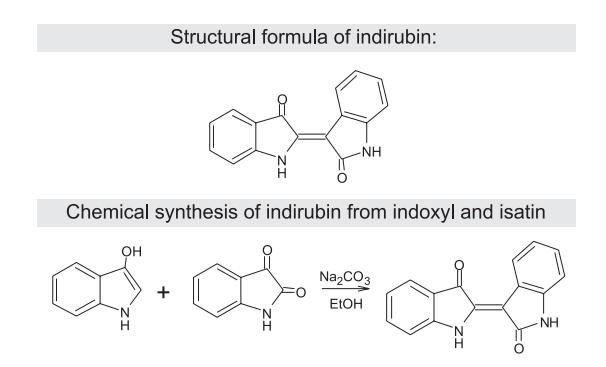
Indirubin is a red dye and an isomer of blue indigo and brown isoindygo. After indigo, it is the second major component of the Indigo naturalis. Chemical properties Indirubin has an intense red color, which arises from the spontaneous dimerization reaction between colorless precursors of the compound ñ indoxyl and isatin. Similarly to indigo, indirubin is only slightly soluble. The chemical formula of this compound is: C16H10N2O2 and it has a molecular weight of 262.26. Natural synthesis Plant species such as Polygonum tinctorium Ait., Baphicacanthus Cusi (Nees) Bremek, Isatis.
indigotica Fort. and Mediterranean snails of the genus Murex, contain natural precursors of indirubin. Those precursors produce three isomers or their derivatives. Compounds containing indigoid bonds do not occur naturally in the pure forms of the above mentioned species. They are products of either an enzymatic reaction of dead material or an acid hydrolysis under the influence of air oxidation. Indirubin can also be formed by either the action of certain bacteria at so called metabolic indican or an indoxyl sulfate. This is a consequense of disorders in tryptophan degradation. Then, this amino acid is present in larger amounts than normal in urine, which can lead to the diagnosis that patient has kidney disease. In such cases, the urine will exhibit a purple-blue color. Those derivatives of indoxyl with a free hydroxyl group, as well as the derivatives of isatin are intermediates in the process of the formation of indigoids.
PHARMACOLOGICAL APPLICATIONS
Qing Dai , a Chinese name for Indigo naturalis, has a salty taste and cooling properties. It affects the functions of the liver, which results in the elimination of toxic heat from the body and a drop of bloodís temperature. Moreover, it relieves convulsions. Accordingly, it is indicated in the increased incidence of epidemic disease, hemoptysis associated with increased temperature, bleeding from the nose, chest pain, mouth ulcers, mumps, inflammation of the throat and larynx, and childrensî convulsions. Orally, Qing Dai is usually used in combination with other herbs from TCM for the following ailments: sun stroke, convulsions associated with epilepsy, cough, chest pain, hemoptysis, phlegm and childrens convulsions. It is forbidden to administer Qing Dai orally to pregnant women. A potential side effect of using the medicine is hay fever. Qing Dai can be used either alone or together with other herbs of Chinese medicine in order to treat: sore throat, eczema, psoriasis, saliva gland, ulcers in the mouth and gingivitis.
Indirubin Due to the low stability of the color, indirubin is rarely used in dyeing of textiles. It has been identified that this compound provides antitumor benefits within the cancer cells of animals. According to studies, indirubin achieves this antitumor effect through the inhibition of DNA synthesis in the tumor cells, while not significantly affecting the inhibition of protein synthesis. Indirubin can form a tertiary compound with the DNA strand and DNA polymerase and thereby block its synthesis. Subsequently, the growth of cancer cells is inhibited. It was also discovered that indirubin affects myeloid cells in patients with cancer.
In China, indirubin is used in conjunction with other substances of plant origin, for the treatment of chronic myeloid leukemia (CML). It is considered that Indigo naturalis is responsible for the anti-leukemia effect. A more detailed analysis has shown that this result was achieved mainly due to the indirubin concentration in the amount of 0.05-0.3% of Qing Dai. Indirubin was also identified in the dried parts of plants from the species Orchidaceae Calanthe R. Br. This species in TCM is considered as a substance which can be used to treat inflammations and bacterial infections. Results suggest that a complex interaction of several mechanisms is the basis of the anti-cancer effect of indirubin. The main mechanism called cyclin-dependent kinases (CDK) involves the inhibition of enzymes, which represents a crucial role in the late phase of the division cycle. Therefore, indirubin and some of its derivatives, block the enzyme complex-dependent kinases called CDK1/cyclin B and CDK5/p25. Indirubin also affects the immune system and has been shown that its long-term use leads to an increased cellular immunity in patients with disorders of this type. It has also been shown to improve the condition of the impaired humoral immunity in the same group of patients.
CONCLUSIONS
Derivatives of indigo and the Indigo naturalis preparation containing indigo dye, have been commonly used in the treatment of various diseases, such as fevers, different kinds of inflammations, or carcinomas. Their pharmacological effects were noticed and adopted by the Chinese into traditional chinese medicine hundreds of years ago. The discovered pharmacological properties confirm the importance of natural dyes in current medical treatment strategies and not only solely for their use in the process of textile dyeing. The current review recommends the collection of all published knowledge currently available on this topic.
By NATALIA STASIAK, WIRGINIA KUKU£A-KOCH* and KAZIMIERZ GOWNIAK
Previous:
RELATED NEWS

