Clinical Efficacy and Safety of Oral indigo naturalis
Categories:
Time of issue:
2023-03-31
Clinical Efficacy and Safety of Oral indigo naturalis
Indigo naturalis is widely used in oral and topical medicines, cosmetics, daily chemicals, paintings and natural dye fields. People may wanna know about its safety for oral use.
Here is a research article about oral indigo naturalis safety for reference:
Clinical Efficacy and Safety of Oral indigo naturalis in Patients with Ulcerative Colitis: A Single-Center Open-Label Prospective Study
Shinya Sugimoto etc
Aims:
Chinese herbal medicine indigo naturalis (also known as Qing Dai) has been used to treat various inflammatory conditions. However, not much has been studied about the use of oral indigo naturalis in the treatment for ulcerative colitis (UC) patients. Studies exploring alternative treatments for UC are of considerable interest. In this study, we aimed at prospectively evaluating the safety and efficacy of indigo naturalis for UC patients.
Methods:
The open-label, prospective pilot study was conducted at Keio University Hospital. A total of 20 patients with moderate UC activity were enrolled.Oral indigo naturalis in capsule form was taken twice a day (daily dose, 2 g) for 8 weeks.
Results:
At week 8, the rates of clinical response, clinical remission, and mucosal healing were 72, 33, and 61%, respectively. The clinical and endoscopic scores, CRP levels, and fecal occult blood results were also significantly improved. We observed 2 patients with mild liver dysfunction; 1 patient discontinued due to infectious colitis and 1 patient discontinued due to mild nausea.
Conclusion:
This is the first prospective study indicating that oral indigo naturalis is effective for inducing remission in patients with moderate UC activity and can be tolerated. Thus, indigo naturalis may be considered an alternative treatment for patients, although further investigation is warranted.
Background
Ulcerative colitis (UC) is an immune-mediated intestinal disease characterized by periods of remission and relapse. The major goal of UC treatment is to induce and maintain remission to alleviate symptoms and promote mucosal healing, which is associated with a favorable long-term prognosis. The current pharmacologic management of UC has relied primarily on mesalamine, corticosteroids, and thiopurines. Corticosteroid dependence and resistance are clinically important problems. Immunosuppressants, such as cyclosporine and tacrolimus, and anti-tumor necrosis factor (TNF)-α agents, such as infliximab and adalimumab, are available for corticosteroid-refractory UC with moderate-to-severe activity; however, prior loss of response or intolerance to these agents and infection due to immuno suppression are increasingly becoming clinical problems. Therefore, studies exploring alternative treatments for UC are of considerable interest.
As described in the Chinese consensus guidelines, some Chinese herbal medicine have anti-inflammatory, anti-diarrheal, and mucosal protection and immuno-suppressive effects and is one of the most frequently chosen alternative therapies for treating UC in China [11, 12] . The herbal medicine indigo naturalis (also known as indigo naturalis) is extracted from plants, such as Indigofera tinctoria , Isatis tinctoria , and Polygonum tinctorium . Importantly, indigo naturalis belongs to a type of indole derivatives, which promote mucosal healing [13–15] ; however, the mechanisms of action of indigo naturalis still remain unknown.
Topical and oral indigo naturalis are used to treat various inflammatory diseases and dermatosis, such as psoriasis [16–18] . Traditionally, indigo naturalis has been used in Chinese medicine to treat UC patients; however, published data on the safety and efficacy of indigo naturalis for UC patients are lacking in the English literature. Yuan et al. [19] have reported that indigo naturalis enemas are associated with significant clinical efficacy and can be used safely in patients with chronic hemorrhagic radiation proctitis. Suzuki et al. [20] have shown that indigo naturalis has significant clinical and endoscopic efficacy in treating UC patients; however, the study was retrospectively conducted, and the dose of indigo naturalis was not precisely established. Furthermore, there are no studies that have prospectively demonstrated the clinical and endoscopic efficacy of oral indigo naturalis for UC patients. We conducted a prospective investigation to show the efficacy of 2 g daily dose of indigo naturalis for UC patients. We believe this work is the first prospective study using single herbal formulas of indigo naturalis for UC patients, demonstrating its clinical efficacy and safety.
Methods
Study Design
This single-center, open-label, prospective pilot study was conducted at Keio University Hospital. The study protocol was reviewed and approved by the Ethics Committee of Keio University School of Medicine (No. 20140253). Written informed consent was obtained from the participants before the commencement of the study and in accordance with the Declaration of Helsinki. This study was pre-registered at the University Hospital Medical Information Network Center (UMIN Clinical Trials Registry, number UMIN000016526; available at http://www.umin.ac.jp/ctr/). The first patient was enrolled in March 2015, and the last patient completed the trial in December 2015.
Patients
Patients who were aged ≥ 20, who had mild-to-moderate UC activity and were diagnosed according to the diagnostic criteria defined by the research group of inflammatory bowel disease in the Ministry of Health, Labor and Welfare in Japan were eligible to participate in this open-label, prospective study. The patients were excluded from the study if they had colitis-associated dysplasia; symptomatic intestinal stenosis; a history of side effect or allergy of Chinese herbal medicine; a need for potential surgery after enrollment in this study; signs of other types of colitis, severe liver, heart, or renal diseases; or a history of malignant diseases, psychosomatic disorders, or infectious diseases. Pregnant or breastfeeding women were excluded from the study. The patients were not treated with leukocytapheresis, prednisolone ≥ 40 mg, adalimumab, tacrolimus or cyclosporine within 2 weeks before the commencement of the study. The patients were not treated with infliximab within 4 weeks before the commencement of the study. Changing the dose of mesalamine or prednisolone within 2 weeks before the commencement of the study was not allowed. Concomitant treatment with thiopurine, started more than 12 weeks before the commencement of the study, was allowed if that dosage had been stable for at least 4 weeks before the study was conducted.
Study Procedures
The patients’ demographic data and disease characteristics were collected. All of the patients underwent colonoscopy within the last week before the intervention and at week 8. The endoscopic disease activity was assessed by trained colonoscopists and was scored according to the Mayo endoscopic score [3] and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) [21] . The clinical disease activity was assessed by trained physicians and was scored according to the Clinical Activity Index (CAI) [7] and Mayo score [3] . The venous blood samples were obtained from each patient at weeks 0 (pretreatment), 4 and 8. The adverse events and concomitant medications were recorded at each visit. All of the patients delivered stool samples (quantitative fecal occult blood tests) within 3 days before the intervention and at week 8.
Intervention
Powdered indigo naturalis imported from China
Primary Efficacy
Endpoint The primary efficacy endpoint was the rate of clinical response at 8 weeks of treatment (last survey point). The clinical response was defined as a decrease in the Mayo score of ≥ 1 in each item. The overall safety was assessed by the patients and investigators during the visit to the clinic.
Secondary Efficacy Endpoint
The secondary efficacy endpoint was the rate of clinical remission and mucosal healing at 8 weeks of treatment (last survey point). Clinical remission was defined as a Mayo endoscopic score of ≤ 1 and other items of Mayo score of 0. Mucosal healing was defined as a Mayo endoscopic score of ≤ 1. The serial changes in Mayo score, partial Mayo score, and endoscopic scores (Mayo endoscopic score and UCEIS) were also assessed. The CRP levels and quantitative fecal occult blood tests at 8 weeks were compared with those at baseline.
Sample Size
This pilot, exploratory study was limited in size, and the scope of this study was to evaluate the efficacy and safety of capsuled indigo naturalis for patients with UC for deciding the sample size of randomized controlled trial in the future. Therefore, the projected sample size was initially 10 patients. After analyzing the data of 10 patients, we added 10 more patients predominantly for safety reasons. Statistical Analyses The changes in scores and laboratory results were evaluated by Wilcoxon signed-rank test. All of the statistical analyses were performed using IBM SPSS Statistic version 22 for Windows (IBM Corp., Armonk, N.Y., USA). Two-sided p values were considered to be statistically significant at a level of <0.05.
Results
Patient Profile at Baseline
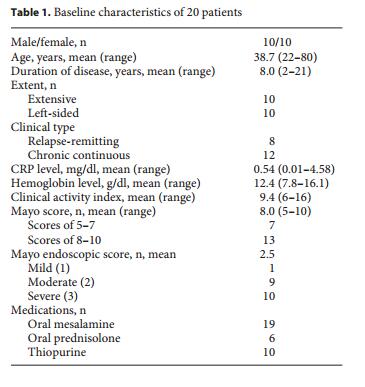
A total of 20 patients (10 male and 10 female) with moderate UC activity were included in the study. The patients’ baseline characteristics are shown in table 1 . At the entries, the mean age was 38.7 (range 22–80 years), and the mean duration of the disease was 8.0 years (range 2–21 years). Each 10 patients were classified as having extensive or left-sided colitis, respectively. The clinical types consisted of 8 patients with relapse-remitting disease course, and 12 with a chronic continuous disease course. At the entries, 19 patients had been treated with oral mesalamine, and 6 patients received oral prednisolone (3–10 mg daily in 5 patients and 30 mg daily in 1 patient). Thiopurines were used in 10 patients (azathioprine, 50–150 mg daily in 8 patients; or 6-mercaptopurine, 5 or 35 mg daily). Three patients were treated with adalimumab for over the past 1 year, but were currently at the condition of secondary failure; they discontinued its use 2 weeks before the entry. Two patients were treated with infliximab for over the past 1 year, but were currently at the condition of secondary failure; they discontinued its use over 4 weeks before the entry. The mean CAI at baseline was 9.4 (range 6–16), and the mean serum CRP level was 0.54 mg/dl (range 0.01–4.58 mg/dl).

Fig. 2. Outcomes of treatment with indigo naturalis. Clinical remission was defined as a Mayo endoscopic score of ≤ 1 and other items of Mayo score of 0. Clinical response was defined as a decrease in the Mayo score of ≥1 in each item.
Clinical Efficacy
As shown in figure 2 , 6 (30%) patients achieved clinical remission, and additionally 7 (35%) patients showed clinical response at 8 weeks. Five (25%) patients did not respond, and 1 (5%) patient discontinued the use of indigo naturalis due to infectious colitis, although she wished to continue this trial due to her initial response to indigo naturalis. One (5%) patient discontinued the use of indigo naturalis due to mild nausea. Actually, the partial Mayo scores were decreased even in the 5 patients who did not respond by definition.
Overall, the clinical scores were significantly decreased by treatment with indigo naturalis. The Mayo score significantly improved from a mean of 7.9 to a mean of 2.7 (p < 0.001; fig. 3 a). Partial Mayo score and CAI also significantly improved from a mean of 5.7 to a mean of 1.4 (p < 0.001; fig. 3 b) and from 9.4 to 3.5 (p < 0.001; fig. 3 c), respectively. Mayo endoscopic score improved from a mean of 2.4 (with a score of 1 in 1 patient, 2 in 8 patients, and 3 in 9 patients) to a mean of 1.2 (with a score of 0 in 3 patient, 1 in 8 patients, and 2 in 7 patients (p < 0.001; fig. 3 d)). The UCEIS improved from a mean of 5.0 (with a score of 2 in 1 patient, 4 in 4 patients, 5 in 8 patients, 6 in 3 patients, and 7 in 2 patients) to a mean of 2.4 (with a score of 1 in 4 patient, 2 in 7 patients, 3 in 3 patients, and 4 in 4 patients (p < 0.001; fig. 3 e)). In figure 4 , clinical endoscopic examples of before and after treatment with indigo naturalis are shown. The overall clinical response rate other than the discontinued case was 72% (13/18), and the overall clinical remission rate was 33% (6/18). Although 3 patients (17%) obtained complete endoscopic remission (Mayo endoscopic score, 0), the rate of mucosal healing (Mayo endoscopic score, 0 or 1) was 61% (11/18).
The serum CRP level was significantly improved from a mean of 0.60 mg/dl to a mean of 0.20 mg/dl (p = 0.02) by treatment with indigo naturalis. The serum albumin level also improved from 3.9 to 4.4 mg/ml (p = 0.001). The mean fecal immunological quantitative test decreased at 8 weeks after treatment with indigo naturalis (2,433 ± 3,002 ng/ ml to 420 ± 533 ng/ml, p < 0.001).
Safety Assessment
No patient developed a severe exacerbation requiring hospitalization over the 8-week period nor did any patient start biologics. Two patients with liver dysfunction, 1 patient with infectious colitis, 2 patients with mild transient headache and 1 patient with mild nausea were reported during the test period. In 1 patient (infectious colitis), the administration of the test agent was ceased at symptom onset (32 days after treatment). Symptoms started after eating broiled meat, and computed tomography revealed thickening of the right side of the colon wall. Due to the typical radiographic pattern of the inflammation, the well-trained radiologist suspected infectious colitis such as Escherichia coli O157 infection. Although fecal culture for the most common intestinal pathogens was negative, this patient was clinically diagnosed with infectious colitis, and causal association with this test agent was considered not likely. In 1 patient, the administration of the test agent was ceased due to mild nausea considered side effects of indigo naturalis (5 days after treatment), and she wanted to change treatment to granulocytapheresis. Two patients with liver dysfunction were observed at week 4 (aspartate aminotransferase (AST)/alanine aminotransferase (ALT) was 47/53 IU/l and 78/82 IU/l, respectively) and other liver tests such as serum bilirubin, alkaline phosphatase and gamma-glutamyl trans peptidase were normal. However, indigo naturalis was continued in these 2 patients, and AST/ALT was not significantly increased at week 8. These liver dysfunctions were reversible after the test was completed, suggesting that they were side effects of indigo naturalis. There were no other adverse side effects reported during the test period.
Fig. 3. Serial changes in clinical scores by treatment with indigo naturalis. a Mayo score (p < 0.001); b partial Mayo score (p < 0.001); c CAI (p < 0.001); d Mayo endoscopic score (p < 0.001); e UCEIS scores (p < 0.001) were significantly decreased at 8 weeks after treatment with indigo naturalis.
Discussion
In this study, we demonstrated that a traditional Chinese medicine indigo naturalis is overall safe and effective for the induction of remission in UC patients. Although the efficacy of oral powdered indigo naturalis has been reported in only one retrospective study [20] , there has been no report of a prospective study of oral indigo naturalis for UC patients. Additionally, we used indigo naturalis in the capsule form, rather than in the powder form, for the purpose of future randomized controlled trial using placebo capsule.
Due to the capsule form, enrolled patients could receive the exact dose of indigo naturalis. This would help in the critical assessment of the safety and efficacy of the medication. The most important finding in our prospective study is that oral capsule indigo naturalis is remarkably effective for inducing remission in patients with moderate UC activity even compared to other well-known approved medicines; thus, indigo naturalis may be a therapeutic option for these patients.
The major goal of therapy in UC is to induce and maintain remission [2] . Although corticosteroids, antiTNF agents and calcineurin inhibitors are effective for inducing clinical remission, these medications have produced no response in several patients. Thus, an alternative treatment for UC patients is needed. Chinese herbal medicine has been widely used to treat UC patients in China. According to a report of Chinese UC patients treated from 1981 to 2000, 20.1% of UC patients were treated with only Chinese herbs, and 59.1% were treated with a combined Chinese and western medicine approach [11] . Thus, Chinese herbal medicine may offer an exciting potential for discovering new agents for UC treatment, and several studies have been conducted along these lines [22, 23] .
Among Chinese medicine, indigo naturalis, extracted from plants, has traditionally been used to treat various symptoms, such as inflammatory condition. The oral or topical products of indigo naturalis are known to be effective and safe treatment options for psoriasis [16–18] . For gastrointestinal diseases, Yuan et al. [19] have reported the clinical efficacy and safety of indigo naturalis enemas in the treatment of chronic hemorrhagic radiation proctitis. However, published data on the efficacy of indigo naturalis for UC patients are mostly lacking in the English literature. In Japan, Suzuki et al. [20] have reported that indigo naturalis is clinically efficacious and safe in treating UC patients, although the data collected were retrospective in nature. Additional reports have been published on the Chinese herbal medicine Xilei-San [24–26] , Kui Jie Qing enema [27] and Fufangkushen colon-coated capsules [28] . These treatments are multiple herbal formulas that partly contain indigo naturalis. Fukunaga et al. [25] have reported the first single-center, randomized, double-blinded, placebo-controlled study showing the efficacy of Xilei-San. In this study, 30 patients with intractable ulcerative proctitis were randomized to receive either Xilei-San or placebo suppositories for 2 weeks. At 2 weeks, the number of patients who achieved clinical and endoscopic remission was significantly higher in the group of Xilei-San vs. placebo (p < 0.04). After 6 months, the rate of recurrence was lower in the group of Xilei-San (Xilei-San 18.2% vs. placebo 83.3%, p < 0.001) [25] . In another randomized, double-blinded study that compared Xilei-San with dexamethasone enemas, a similarly significant clinical, histological and endoscopic response compared with the baseline in the 2 groups was achieved [26] . Importantly, no significant adverse side effects were observed in either study [25, 26] . In the dextran sulfate sodium-induced colitis rat model, topical treatment with Xilei-San attenuates colitis by degrading proinflammatory mediators and promoting mucosal repair [29] . Although these studies showed the efficacy of Xilei-San for UC patients, it still remains unknown whether indigo naturalis included in a mixture of Xilei-San is in fact a key effective component or not.
In our study, we used single herbal formulas indigo naturalis in the capsule form because the patients would not have been able to take an accurate and appropriate dose of indigo naturalis in the powder form. To assure the safety and accuracy of our study results, we selected the capsule form of indigo naturalis. Additionally, the capsule form is useful in the setting of placebo-controlled studies and for enabling future drug approval. Since indigo naturalis appears dark blue, it was difficult to perform a placebo-controlled trial with the powder form. According to the previous study [20] , we set the daily dose of 2 g, although a daily dose of 3 g is recommended in patients with psoriasis [18] . The adverse side effects of indigo naturalis are typically diarrhea, abdominal pain, nausea, vomiting, liver dysfunction, cutaneous symptoms, leukocyte decrease, dizziness and headache [18, 30] . We were especially concerned about liver dysfunction because of the well-known toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the aryl hydrocarbon receptor (AhR) signaling [13] . The clinical manifestations of TCDD exposure include progressive liver or renal failure, emphysema, and myocardial degeneration [13] . In our study, severe liver dysfunction was not observed. However, 10% of reversible liver dysfunction may be considered to be a high rate. Fearing adverse events occurring after long-term use in the future, we decided to lower the dose of indigo naturalis and conducted a randomized, dose-dependency, clinical trial for UC patients. At the moment, the differential emergence of adverse effects between TCDD and natural ligands such as indigo naturalis remains unknown; there should be a high alert on these issues, although we use indole derivatives such as indigo carmine for endoscopic dye in daily practice.
We demonstrated significant clinical and endoscopic response in UC patients treated with oral indigo naturalis in this study. We considered that the mechanisms of efficacy of indigo naturalis may be associated with AhR signaling. An important group of natural AhR ligands is indole, such as indole-3-aldehyde (3-IAld), indole-3-acetaldehyde, indole-3-acetic acid and indole-3-lactic acid, which can be generated by the bacterial metabolism of tryptophan and are also derived from the metabolism of dietary intake [13, 14] . Interleukin (IL)-22, a member of the IL-10 cytokine family, is the primary downstream product of AhR activation by 3-IAld stimulation in innate lymphoid cells type 3 (ILC3). Commensal bacteria utilize tryptophan as an energy source and produce 3-IAld [15] . While AhR has been shown to promote IL-17 expression in vitro [31, 32] , the AhR signaling pathway suppresses Th17 cell differentiation in vivo [15, 33] . In the gut, IL-22 regulates intestinal immune homeostasis and mucosal wound healing by the activation of epithelial signal transducer and activator of transcription 3 [34] . IL-22 also regulates the release of antimicrobial peptides, such as calprotectin, S100A8, and S100A9, which have anti-inflammatory effects [35] . Therefore, AhR-related compounds have a protective mechanism implemented through the 3-IAld/ AhR/IL-22/IL-10 axis that can be therapeutically useful in treating UC patients. Another mechanism of the efficacy of indigo naturalis has been indicated through the anti-inflammatory effects because of its ability to suppress superoxide generation [36].
Chinese herbal medicine is usually administered as multiple herbal formulas in China, and the use of single herbal formulas has not reached an expert consensus [18] . However, purified natural products as ingredients are preferable, rather than crude extracts, to manufacture high-quality medications. In fact, crude drug is usually difficult to get approved as a drug in Japan and in Western countries. Our study intended to use single herbal formulas rather than multiple herbal formulas. A considerable barrier to the acceptance of Chinese herbal medicine by conventional physicians has been the lack of scientific explanation for their possible efficacy and safety. Studies of pharmacokinetic and medicinal interactions are required to determine their efficacy and safety. Indigo naturalis contains natural ingredients, such as indigo, indirubin, isoindigo, and tryptanthrin [36] . To identify the compound of indigo naturalis, which is effective for treating UC, is an important agenda for future study. The project aimed at exploring the compound related to indigo naturalis is already in process. Natura-alpha (meisoindigo; N-methyl- Δ3,3 ′ -dihydroindole-2,2 ′ diketone), an indirubin derivative, is a synthetic small molecule oral compound, which is believed to inhibit the expression of pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-α, and to block inflammatory cytokines by stimulating the production of IL-10 [37] . A phase II trial (ClinicalTrials.gov identifier: NCT01216280) of Natura-alpha is currently underway for the treatment of moderate-to-severe UC patients.
According to this study, although indigo naturalis seems to be attractive in terms of the safety and efficacy aspects, it has several limitations. First, this was a single-center, uncontrolled study, with a less number of patients. It is well known that there is a particularly high rate of placebo effect in UC [38] . Patient masking and direct comparison with a current treatment control group are lacking. However, it is critical to assess the safety of capsuled (exact 2 g daily) indigo naturalis in the small study to conduct large randomized controlled trial in the future. From the results of our pilot study, we have recognized that liver dysfunction, headache and digestive symptom should be cautioned in patients with 2 g daily of indigo naturalis and the sample size can be desirable. The following clinical questions can be posed concerning the use of indigo naturalis. Can safety and efficacy be maintained when combining indigo naturalis with conventional treatment with components such as mesalamine, corticosteroids, thioprines, and anti-TNFα agents? Can indigo naturalis be used for treating patients with Crohn’s disease or Behçet’s disease? Can indigo naturalis be used for maintenance of remission? To answer these questions, further investigation is warranted. We might have considered treatment with corticosteroid or tacrolimus if 20 patients had not registered for enrollment in this study; thus, indigo naturalis was considered an alternative treatment to avoid the use of corticosteroids or other immunosuppressants.

Fig. 4. Clinical endoscopic examples of before and after treatment with indigo naturalis (representative 5 cases). a , c , e , g , i Before treatment with Qing-Dai; b , d , f , h , j after 8 weeks of treatment with indigo naturalis.
Conclusion
In conclusion, we prospectively showed significant clinical and endoscopic response in UC patients treated with oral indigo naturalis at 8 weeks. Thus, oral capsuled indigo naturalis is effective for inducing remission in patients with moderate UC activity and can be tolerated, although further investigation is required.
Source
1.http://www.ncbi.nlm.nih.gov/pubmed/26959688
Back To Previous
RELATED NEWS

